

leaving group: In organic chemistry, the species that leaves the parent molecule following a substitution reaction.Īlcohols are organic compounds in which the hydroxyl functional group (-OH) is bound to a carbon atom.carboxylic acid: Any of a class of organic compounds containing a carboxyl functional group-a carbon with a double bond to an oxygen and a single bond to another oxygen, which is in turn bonded to a hydrogen.
aldehyde: Any of a large class of reactive organic compounds (R♼HO) having a carbonyl functional group attached to one hydrocarbon radical and a hydrogen atom.alkane: Any of the saturated hydrocarbons-including methane, ethane, and compounds with long carbon chain known as paraffins, etc.- that have a chemical formula of the form CnH2n+2.Alcohols also can become deprotonated in the presence of a strong base. In chemical reactions, alcohols often cannot leave the molecule on their own to leave, they often become protonated to water, which is a better leaving group.This is attributed to the difference in electronegativity between the carbon and the oxygen atoms.
This leads to higher boiling points compared to their parent alkanes.
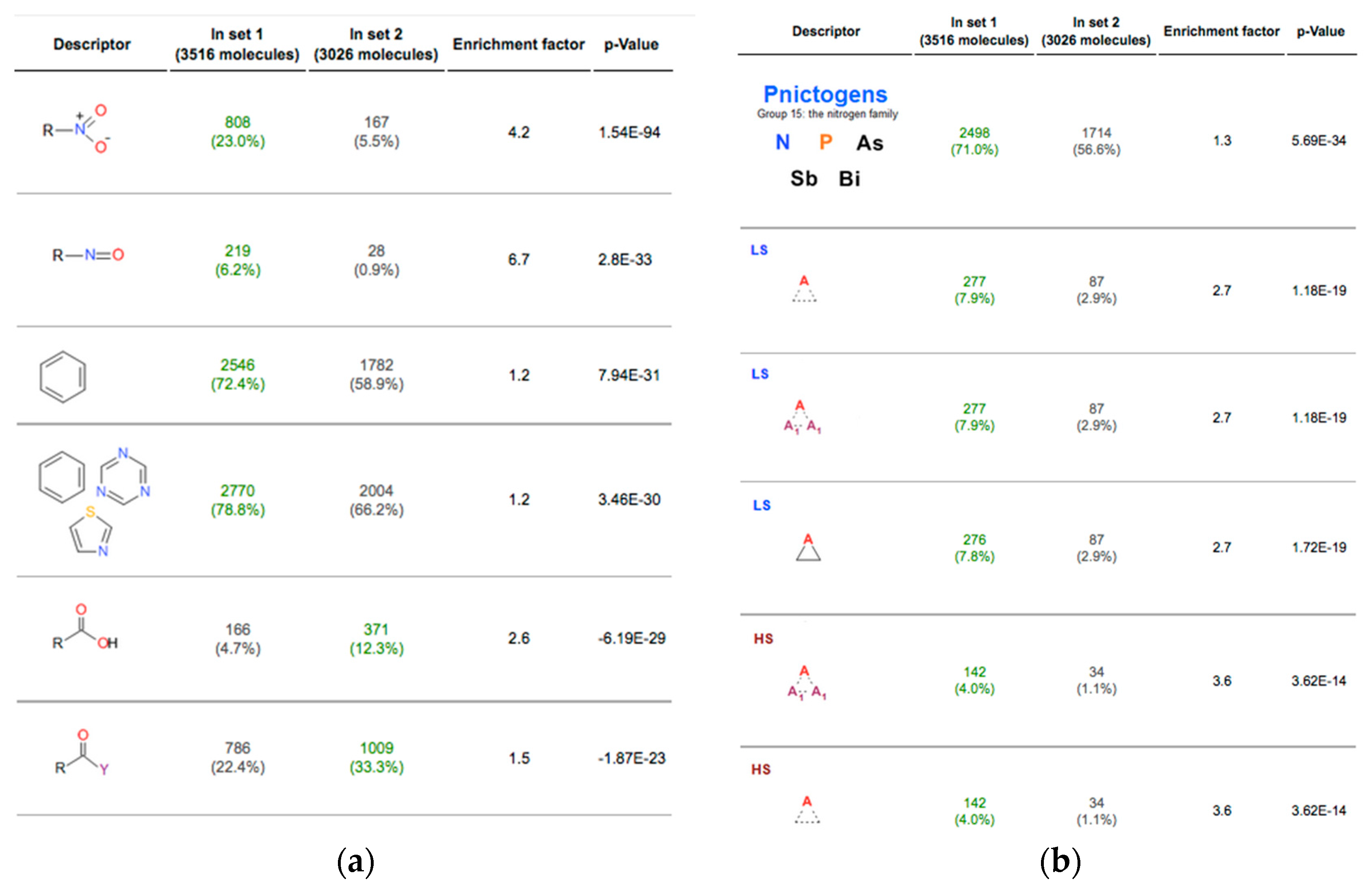
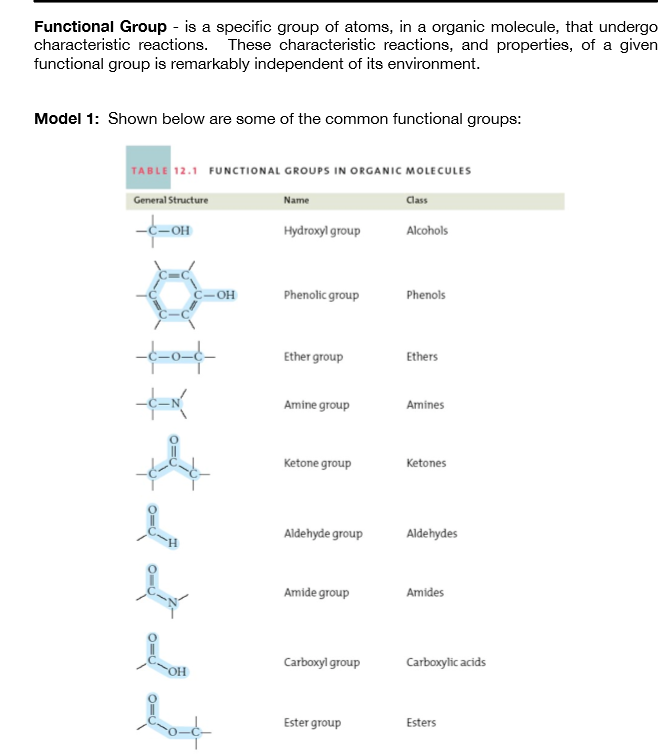
Due to the presence of an -OH group, alcohols can hydrogen bond. It is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds. In organic chemistry, the most common functional groups are carbonyls (C=O), alcohols (-OH), carboxylic acids (CO 2H), esters (CO 2R), and amines (NH 2). In materials science, functionalization is employed to achieve desired surface properties functional groups can also be used to covalently link functional molecules to the surfaces of chemical devices. Through routine synthesis methods, any kind of organic compound can be attached to the surface. Functionalization refers to the addition of functional groups to a compound by chemical synthesis. Often, compounds are functionalized with specific groups for a specific chemical reaction. Alkyl chains are often nonreactive, and the direction of site-specific reactions is difficult unsaturated alkyl chains with the presence of functional groups allow for higher reactivity and specificity. Functional Groups and Reactivityįunctional groups play a significant role in directing and controlling organic reactions. They can be classified as primary, secondary, or tertiary, depending on how many carbon atoms the central carbon is attached to. Similarly, a functional group can be referred to as primary, secondary, or tertiary, depending on if it is attached to one, two, or three carbon atoms.Ĭlassification of alcohols: Alcohols are a common functional group (-OH). The first carbon atom that attaches to the functional group is referred to as the alpha carbon the second, the beta carbon the third, the gamma carbon, etc. The atoms of a functional group are linked together and to the rest of the compound by covalent bonds. Functional groups also play an important part in organic compound nomenclature combining the names of the functional groups with the names of the parent alkanes provides a way to distinguish compounds. The same functional group will behave in a similar fashion, by undergoing similar reactions, regardless of the compound of which it is a part. In organic chemistry, a functional group is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound. functionalization: Addition of specific functional groups to afford the compound new, desirable properties. functional group: A specific grouping of elements that is characteristic of a class of compounds, and determines some properties and reactions of that class. Functional groups can be used to distinguish similar compounds from each other. Functional groups will undergo the same type of reactions regardless of the compound of which they are a part however, the presence of certain functional groups within close proximity can limit reactivity. Functional groups are often used to "functionalize" a compound, affording it different physical and chemical properties than it would have in its original form.







 0 kommentar(er)
0 kommentar(er)
